The Wicked Witch of the West, Phase Changes, and Building Science

Which takes more heat — melting a pound of feathers or raising the temperature of a pound of feathers from the melting to the boiling point? OK, let me rephrase that. If the Wicked Witch of the West melts at a temperature of 45° C and her current temperature is 35° C, how much heat does it take to get her to the melting point and then to melt her completely?
Whoa! Let’s try that again. Remember back in those science classes when you learned about phase changes, those transitions among solid, liquid, and gas that substances undergo? They’re pretty important in building science, too. In fact, they show up all over the place in building science, so let’s take a closer look.
The science of phase changes
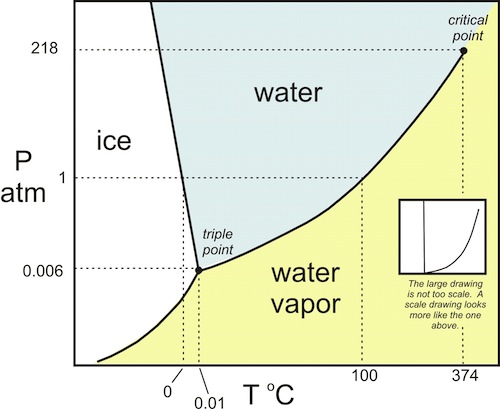
The graph below shows the behavior of water at various temperatures and pressures. At a given pressure, say 1 atmosphere, the phase of water changes from ice to liquid to vapor as you increase the temperature. Yeah, yeah. I know. This is pretty basic stuff, but hear me out.
Temperature and heat aren’t the same thing, though, so the really interesting thing here is what happens with the amount of heat it takes to raise the temperature of a substance and to make it change phase. Again, let’s look at water.
When you add heat to water, starting with it in the solid phase, first it causes the temperature to rise until it reaches the melting point. Then, while the ice is melting, the temperature stays constant until it’s all melted. Once you have all liquid water, the heat added starts raising the temperature again, this time until it reaches the boiling point. Then the water boils at constant temperature. The last stage is that the steam temperature rises as you add more heat.
This is where it starts getting interesting. Notice the numbers on that graph for the amount of heat it takes to raise the temperature of liquid water and then the amount of heat it takes to melt ice and boil liquid water.
It takes 334 kiloJoules (kJ) to melt one kilogram of ice. Then it takes another 419 kJ to raise the temperature of that kilogram all the way from the melting point to the boiling point. Then it takes 2260 kJ of heat to boil that kilogram completely. Wow! That’s a lot of heat compared to the other things happening with water. It turns out to be pretty important, too.
Phase changes in building science
The amount of energy involved in water going between the liquid and vapor phases plays a big role in air conditioning in humid climates. As you probably know, water also is probably the most important single substance in building science. It also proved crucial to Dorothy as she battled the Wicked Witch of the West. It’s wise to understand its properties. Let’s look at a list of some of the notable phase changes that affect buildings, mostly involving water.
- Water vapor condenses on windows in winter.
- Water vapor adsorbs onto or absorbs into sorptive materials (e.g., wood, drywall). Bill Rose’s book, Water in Buildings, is an excellent source for understanding this process.
- Water vapor condenses on the evaporator coil in an air conditioner, dehumidifier, or refrigerator.
- Water evaporates into the air from cooking, bathing, cleaning, and sweating.
- Water evaporates into the air from a humidifier.
- Refrigerant absorbs heat and evaporates in the evaporator coil of an air conditioner, dehumidifier, or refrigerator.
- Refrigerant releases heat and condenses in the condensing coil of an air conditioner, dehumidifier, or refrigerator.
- Snow melts on a roof, drains down to the eave, and refreezes, forming that wonderful bane of cold climate homes known as the ice dam.
There’s a lot of building science to discuss in that list. I’ve already talked about a lot of it here in the Energy Vanguard Blog, but there’s so many directions to go with this stuff, you’ll definitely see more.
Harnessing phase changes for energy efficiency
 Let’s finish up here with a brief discussion of the good things we can do with phase changes. Last year I wrote about Appalachian State University’s Net Zero Energy home for the Solar Decathlon, and one of the ideas they used was to store solar energy in a phase change material. During the day, a solar thermal collector on the roof would capture solar energy and transfer it down to the box you see here. The material was solid before and would melt when the heat arrived. Using the heat in the home would cause the material to solidify again.
Let’s finish up here with a brief discussion of the good things we can do with phase changes. Last year I wrote about Appalachian State University’s Net Zero Energy home for the Solar Decathlon, and one of the ideas they used was to store solar energy in a phase change material. During the day, a solar thermal collector on the roof would capture solar energy and transfer it down to the box you see here. The material was solid before and would melt when the heat arrived. Using the heat in the home would cause the material to solidify again.
At the Passive House Institute of the US (PHIUS) conference last September, I spoke with a vendor whose company makes phase change materials to go into building assemblies. The idea is to capture the heat in the phase change to slow the rate of heat loss in winter or heat gain in summer. They can even tune the materials so that the phase change happens at the best temperature for a given building assembly and climate zone. Cool idea, but not without its drawbacks.
Of course, the ultimate phase change materials we use in homes are the refrigerants in our refrigerators, air conditioners, heat pumps, and dehumidifiers. As the refrigerant moves through the system, it cycles between liquid and vapor phases. The phase changes happen in the two coils (the evaporator coil and the condensing coil), and allow these machines to transfer heat from one place to another, or, in the case of dehumidifiers, to take water vapor out of the air.
As you can see above, the Wicked Witch of the West underwent a rather severe phase change. I’m not sure if Dorothy went on to become a building scientist upon her return to Kansas, but she evidently had some good insight into the behavior of water!
Check out my followup article on this topic: Latent Heat is the Wicked Witch of the South. I think you’ll like it.
Related Articles
The Magic of Cold, Part 1 – How Your Air Conditioner Works
Why Is Fall a Good Time of Year for Window Condensation?
Guest Post: What Causes Ice Dams and Icicles? by Nate Adams
Image of phase diagram from Carleton University. Image of temperature vs. heat graph from Montana Science Partnership.
This Post Has 4 Comments
Comments are closed.


Another nice post Allison!
Another nice post Allison! Wonder if my mother would understand it – or is it just fun for us geeks…
So, what 72f phase change materials are there, and what do THOSE curves look like?
Phase change materials are a
Phase change materials are a nice way to emulate more thermal mass than you have, provided one stays within the phase change range. One company that makes PCM (Phase Change Materials) is Phase Change Energy Solutions http://www.phasechange.com . They make thermal storage mats for commercial buildings. It’s unclear to me whether it is cost-effective to use, but still interesting.
Ted K.:
Ted K.: Thanks. I’ve barely scratched the surface in my learning about phase change materials so I can’t answer your question about specific properties.
Dale S.: “Phase change materials are a nice way to emulate more thermal mass than you have.” That’s a great way to explain what PCMs do! And thanks for the link, which is clickable here: Phase Change Energy Solutions.
PCM was installed on side of
PCM was installed on side of a Passive House duplex here in Portland, OR known as the Trekhaus. Portland State University (PSU) is studying it’s affects against the side of the duplex that doesn’t have the material installed. For more info check out their blog: https://sites.google.com/site/trekhauspdx/