The Ol’ Beer Can Pressure Crunch

Air has weight. Air can move things, like giant wind turbine blades. Air can also crush things. I first got to see the power of air in my introductory physics class in college. The professor, Dr. Jeffrey Trahan, showed the class an empty steel can just like the one in the photo below. He then did a little manipulation of the physical parameters. The result was that the can, made with a fairly heavy gauge of steel, got crushed right before our eyes. I did this demonstration in my own classes later on, but now there’s an easy way for anyone to do it. I call it the ol’ beer can pressure crunch.

Balanced and unbalanced pressures
When you hold out your hand, the weight of the air above it is about 200 pounds. You don’t feel it, though, because that weight causes the air around your hand to exert pressure in all directions. That means the 200 pounds pushing down on your hand is matched by 200 pounds pushing up. Thus, you don’t have to do any work to hold up that air. It’s actually the air below your hand holding it up.
To see the effect of air pressure, we have to get it to push fully on one side of an object with a reduced pressure on the other side. That’s how Dr. Trahan crushed the steel can. And it’s how I crushed the aluminum beer can in the lead photo. Here’s video showing the beer can pressure crunch, with a short explanation at the beginning.
Psychrometric trickery
The way I (and Dr. Trahan) achieved a pressure difference between the inside and outside of the can was through a little psychrometric trick called condensation. I put a little bit of water in the can and and then set the can on a hot burner on my stove. Once the water got to a full boil and I saw steam escaping from the top, I screwed the cap on and removed it from the heat.
At first, the pressure inside the can is equal to the pressure outside, and nothing happens. The air inside, however, is different from the air outside. It has a much higher percentage of water vapor in it than the air outside. But that’s a temporary state.
As the can cools, so do the air and water vapor inside the can. The water vapor starts condensing on the inside of the can. As the water vapor leaves the air, the pressure inside the can decreases. That puts a pressure difference across the can, with the outside pressure getting getting more and more of an advantage. At a certain point, it crushes the can, as you saw in the video.
Other cool pressure tricks
You can see dozens, maybe hundreds, of videos of this demonstration on YouTube. Most of them do it with a pop-top can, which the demonstrator quickly removes from the heat and dunks upside down into a bowl of ice water. I like the slow approach better, and screw-top beer cans work perfectly!
I mentioned that my first experience with this demonstration was with a heavier gauge steel can, but it can go even further. Here’s a video showing the same thing happen to a 55 gallon drum. Yes, really!
This isn’t the only way crush a can with air pressure, of course. You could simply use a vacuum pump to pump the air out, and the same thing would happen.
Applications to building science
Using regular atmospheric pressure to crush cans of any size is fun, but how does this phenomenon relate to buildings? First, for any air to leak into or out of a building, you need two things: a pathway and a pressure difference. In the photo below, you can see two pathways between the conditioned space below and an unconditioned attic. The pressure could be higher on either side, so you could be getting nasty, hot, humid attic air leaking into your house, or you could be losing your nice warm air to the attic.

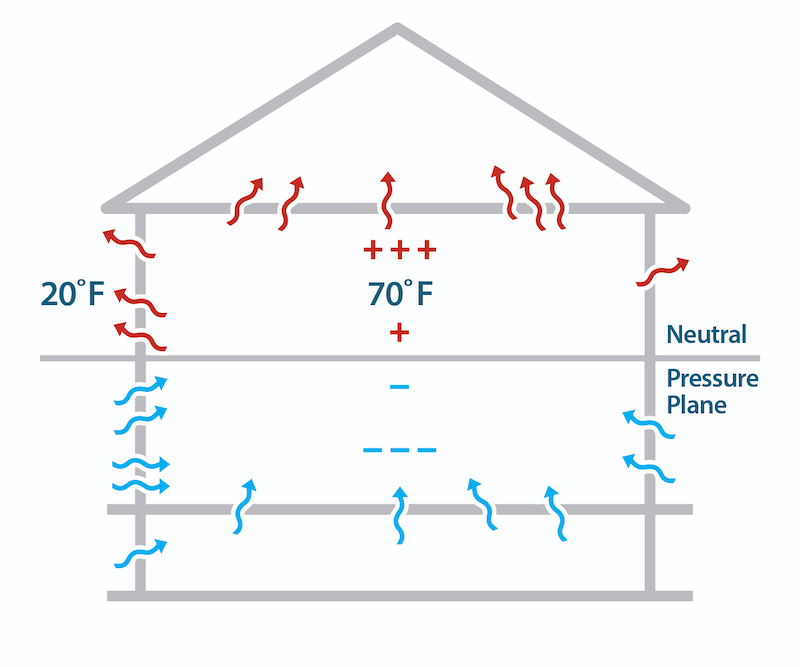
The other aspect of air leakage is the pressure difference. The three main ways they arise across a building enclosure are through the stack effect, wind, or mechanical systems (e.g., exhaust fans). The stack effect is what causes warm air to rise. And that goes back to the weight of all that air sitting on the palm of your hand.
The air pressure on the outside of a building is higher at the bottom than at the top because air pressure decreases with height. The indoor air is usually at a different temperature from outdoors, and that causes the indoor air to be at a different pressure, too. The result is that in winter, air leaks into the house at the bottom and leaks out at the top.
There’s your little beer can pressure crunch building science lesson for the day! And by the way, Miller Lite isn’t my beer of choice. (Sorry, KG.) I don’t always drink beer but when I do, it’s usually a craft beer, like this nice dark malty beer from Three Taverns Brewery that I drank at my favorite drinking establishment, the Brick Store Pub in downtown Decatur, Georgia.

Cheers! Prost! Sláinte!
Allison A. Bailes III, PhD is a speaker, writer, building science consultant, and the founder of Energy Vanguard in Decatur, Georgia. He has a doctorate in physics and writes the Energy Vanguard Blog. He also has a book on building science coming out in the fall of 2022. You can follow him on Twitter at @EnergyVanguard.
Related Articles
What Is Pressure? – Understanding Air Leakage
Heat Rises…and Falls — Stack Effect, Air Movement, & Heat Flow
The 3 Rules of Air Leakage (Plus a Bonus!)
Comments are moderated. Your comment will not appear below until approved.
This Post Has 5 Comments
Comments are closed.

Allison,
I like the beer can crush! That was cool Man! That hole was not, and neither is unchecked stack effect. Time to look in the attic again.
Thanks,
Tommy
Outstanding explanation! I did this every year for our Scouts.
Another great explanation. We deal with a lot of invisible things: vapors, spores, radon, building pressure, thermal conductivity, …………..
Thanks for making the invisible a little more visible.
Barry Westbrook, CIH, PE (KY)
DocAir Building Solutions
Of course, with a vessel engineered the right way – like a Kerr jar – this is how canning works: heating at or near boiling fills the airspace at the top with water vapor, driving out other constituents of air like oxygen, then after being capped, the cooling and condensing leaves the airspace not only largely dehydrated but largely deoxygenated. Course then you need a screwdriver or other leveraging device to pull off the cap!
Allison,
I always learn something from you, so I read every post. Thanks for educating so many people w/your blog. I often send them to my wife so she knows I’m not crazy when I tell her why we should do this or that. An induction stove is in your future. We love ours and now are all electric.