What IS Heat Anyway? – Building Science Fundamentals

 Back in grad school, I was taking a class in surface physics, and my friend Steve asked, “What is temperature?” On first hearing that question, you might think it’s so obvious that it doesn’t even bear asking, and anyone who does give voice to it must be an idiot. Right?
Back in grad school, I was taking a class in surface physics, and my friend Steve asked, “What is temperature?” On first hearing that question, you might think it’s so obvious that it doesn’t even bear asking, and anyone who does give voice to it must be an idiot. Right?
Back in grad school, I was taking a class in surface physics, and my friend Steve asked, “What is temperature?” On first hearing that question, you might think it’s so obvious that it doesn’t even bear asking, and anyone who does give voice to it must be an idiot. Right?
Wrong! Perhaps the most important lesson I learned in my seven years of grad school was to ask questions, even about things that seem so basic as not to need questioning. There a lot of insights and deeper understanding available when you start questioning your basic beliefs.
Energy, temperature, and heat
Let’s look at a question related to Steve’s: What is heat? All of us who work or play in the world of building science talk about it, so it’s a good idea to know a little about this basic concept.
Actually, the two questions are related. Heat and temperature go together, but they’re not the same thing. Before we can understand heat and temperature, though, we’ve got to talk about thermal energy. It sounds like thermal energy and heat might be the same thing, too, but they’re not.
Thermal energy is the combined  energy of all the jiggling going on in the molecules of an object.
energy of all the jiggling going on in the molecules of an object.
Temperature is a measure of the average kinetic energy (energy of motion) of the molecules.
Heat is a measure of how much thermal energy transfers from one object to another because of a temperature difference.
Of the three quantities, temperature is the one we have direct access to. It’s the one we learn about at an early age, when we touch something hot and get burned. It’s also the key to understanding heat because heat flows only when there’s a temperature difference (or during phase changes and combustion, but let’s stick to things don’t change form today). You don’t learn any life lessons by touching something that’s the same temperature as your skin because no temperature difference means no heat flow.
Go with the flow
Really, the term “heat” should be used only in conjunction with “flow” or “transfer” because it’s what happens when there’s a temperature difference, and one body (at a higher temperature) transfers thermal energy to another (at a lower temperature). We don’t really care about thermal energy in building science because heat flow is what matters.
We can easily calculate the amount of heat that we lose through the building enclosure (walls, floors, and ceilings that separate conditioned from unconditioned space) by conduction on a cold night. The equation is:
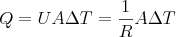
where Q is the amount of heat flow (in BTU per hour), U is the heat transfer coefficient, A is the area, and ΔT is the temperature difference. For more on the details of this equation, see my Flat or Lumpy article.
Now, where this really comes in handy is when you want to do some energy modeling or heating and cooling load calculations for the purpose of sizing the heating and cooling equipment. You use that formula for all parts of the building enclosure, add in your other loads (ducts, people, appliances…), and you can find out how much energy a home uses or needs for heating and cooling. It’s all about heat flow, which is all about temperature difference, which is all about thermal energy.
Let me emphasize one point here before I end: Heat flows only when there’s a temperature difference, and it naturally flows from a warmer body to a cooler body. You can pump it the other way, and that’s exactly what refrigerators, air conditioners, and heat pumps do. But the Second Law of Thermodynamics says heat wants to flow from warmer to cooler bodies.
That law is one of the most important and interesting in all of physics, and we could spend the rest of the year engrossed in a discussion of the Second Law topics of entropy and the arrow of time, the heat death of the universe, why some processes are irreversible, and how it’s possible, according to statistical mechanics, for all the air in the room to be suddenly in one little corner, leaving you gasping in your chair as you read this blog. But that’ll have to wait for another time.
For now, the big takeaway is that when we talk about heat, we’re talking about a process. It’s not a quantity that’s in something. It’s a quantity that moves from one (warmer) thing to another (cooler) thing.
Lead photo by Energy Vanguard. Second photo by lorda from flickr.com, used under Creative Commons license.
This Post Has 3 Comments
Comments are closed.

Funny this post comes up
Funny this post comes up while I’m reading Manual J. The discussion about Dry-Bulb Temperature, it it says, “If a substance has no molecular activity, it has no heat content and has no temperature”. This happens at 0 degrees K. Until then, I never really though about it!
Well, that’s roughly correct,
Well, that’s roughly correct, Sam, but not completely. Strictly speaking, an object doesn’t contain heat. It contains thermal energy and potential energy of various sorts and something else called zero point energy. When thermal energy moves from one body to another, it’s heat, or heat flow.
Also, because of the zero point energy, removing all the thermal energy and all the potential energy still doesn’t get you to absolute zero (0 K). That’s the Third Law of Thermodynamics, which says getting to absolute zero is impossible.
“we could spend the rest
“we could spend the rest of the year engrossed in a discussion of the Second Law topics of entropy and the arrow of time…”
Bring it on!